Abstract
Introduction
Post-transplant cyclophosphamide (PTCy) for controlling alloreactivity after allogeneic hematopoietic stem cell transplantation (HSCT) is an effective prophylaxis for acute and chronic graft-versus-host disease (GVHD). HLA-haploidentical HSCT using PTCy is a promising option for patients with hematological malignancy who require allo-HSCT and lack an immediately available HLA-identical related or unrelated donor. In contrast, a relatively high relapse rate was reported in HLA-haploidentical HSCT using PTCy with non-myeloablative conditioning compared to HLA-matched related or unrelated HSCT using conventional GVHD prophylaxis. The effects of GVHD have been shown to correlate partially with those of graft-versus-leukemia (GVL), and therefore, PTCy treatment might contribute to the increase in relapse. However, the rationale for the high relapse rate in HSCT using PTCy compared to conventional HSCT remains poorly understood. To address this issue, we explored the effect of PTCy on survival, leukemia progression and immune reconstruction in a murine transplant model.
Material & Methods
Twelve-week old C57BL/6 (B6) mice were irradiated with 5 Gy in one fraction on day 0 and transplanted with 1.0 × 107 spleen cells and 1.0 × 107 bone marrow cells from Balb/c mice. All mice were purchased from Japan SLC Inc. and maintained in a pathogen-free environment. Mice consumed normal chow and autoclaved hyperchlorinated water (pH 4) after the transplantation. Mice were not treated (control) or received cyclophosphamide 100 mg/kg at days 3 and 4 intraperitoneally. A leukemia cell line that was derived from the B6 bone marrow and transformed with the BCR/ABL fusion gene (BA-1) was used for leukemogenic experiments. One million BA-1 cells were intravenously infused into the recipients 25 (±4) days after transplantation with no additive treatment. We continuously examined weight, GVHD score, peripheral blood cell counts, and peripheral blood cell prolife of the recipient mice, and finally investigated spleen and bone marrow cells. The chimeric balances among host-residual H-2Db+ cells and donor graft-derived H-2Dd+ cells in CD3+ T cells were monitored. To evaluate the function of activated T cells in the spleen, CD62L+, CD44+, and PD-1+ marker levels in CD8+ and CD4+ cells were quantitatively monitored at various time points. GVHD scoring was based on weight loss, posture, activity, fur texture, and skin integrity (severity score 0 to 2 for each variable, maximum index 10) as previously reported. Leukemia progression was defined by the presence of 0.05% BA-1 cells in peripheral blood mononuclear cells as detected by GFP expression. All experimental procedures were approved by an institutional review committee of the Osaka City University.
Results
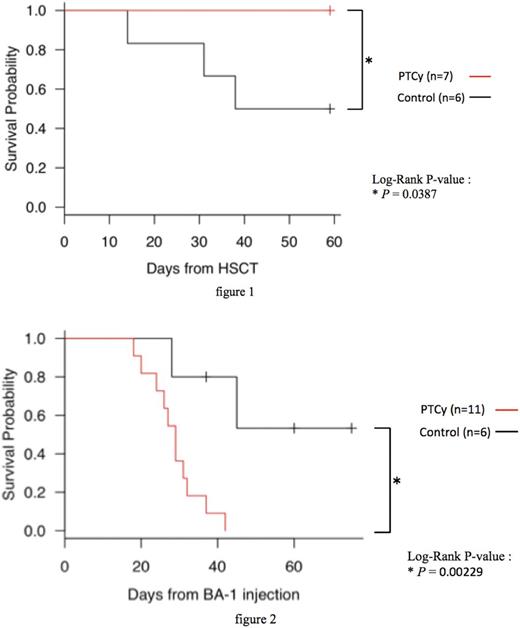
Control B6 recipients developed severe weight loss and GVHD after transplantation and died of GVHD at around day 60, whereas the recipients with PTCy had significantly improved weight, GVHD score, and survival compared to control mice (figure1). These results were comparable to those of previous reports. In the leukemogenic experiment, the control recipients showed significantly longer survival than the ones with PTCy (figure2). Recipients with PTCy had a higher rate of leukemia progression compared to the control at day 30 after BA-1 injection, although there were no significant differences in the chimeric balances among donor graft-derived H-2Dd+ T cells both before and at day 7, 14, 21, 28, and 35 after BA-1 injection. Prior to BA-1 injection, CD62L+ CD44- naïve CD8+/CD4+ T cell number was low, and CD62L- CD44+ effector memory CD8+/CD4+ T cell number was high in the spleen of the control recipients compared to that in PTCy recipients. Moreover, the numbers of CD44+ PD-1+ memory CD8+/CD4+ T cells were also overwhelmingly high in the spleen of control recipients. These results showed that use of PTCy after allo-HSCT promoted T cell tolerance to alloantigens by eliminating antigen-exposed T cells and reduced the probability of rejection in leukemia as a consequence.
Conclusion
Our data indicated that PTCy treatment after allo-HSCT contributes to impaired GVL effects by reducing the number of antigen-exposed T cells and improves GVHD regulation in a murine stem cell transplant model.
Hino: Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal